
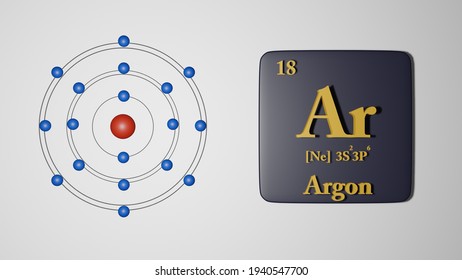
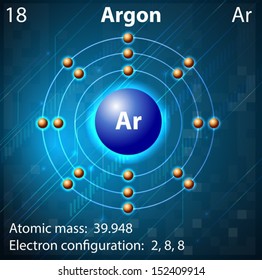
Argon isotopes are used as precursors in the production of radioisotopes. Ar-40 and Ar-38 are used in the production of radioactive K-38 which can be used as a blood flow tracer. Ar-40 is used in the production of radioactive Ar-41 which is used to trace gas flows. Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in the Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times. In order to write the Argon electron configuration we first need to know the number of electrons for the Ar atom (there are 18 electrons). When we write the configuration we'll put all 18 electrons in orbitals around the nucleus of the Argon atom. In writing the electron configuration for Argon the first two electrons will go in the 1s orbital.
The Element Argon
[Click for Isotope Data]
Atomic Number: 18
Atomic Weight: 39.948
Melting Point: 83.80 K (-189.35°C or -308.83°F)
Boiling Point: 87.30 K (-185.85°C or -302.53°F)
Density: 0.0017837 grams per cubic centimeter
Phase at Room Temperature: Gas
Element Classification: Non-metal
Period Number: 3
Group Number: 18
Group Name: Noble Gas
What's in a name? From the Greek word for inactive, argos.
Say what? Argon is pronounced as AR-gon.
History and Uses:
Argon was discovered by Sir William Ramsay, a Scottish chemist, and Lord Rayleigh, an English chemist, in 1894. Argon makes up 0.93% of the earth's atmosphere, making it the third most abundant gas. Argon is obtained from the air as a byproduct of the production of oxygen and nitrogen.
Argon is frequently used when an inert atmosphere is needed. It is used to fill incandescent and fluorescent light bulbs to prevent oxygen from corroding the hot filament. Argon is also used to form inert atmospheres for arc welding, growing semiconductor crystals and processes that require shielding from other atmospheric gases.
Once thought to be completely inert, argon is known to form at least one compound. The synthesis of argon fluorohydride (HArF) was reported by Leonid Khriachtchev, Mika Pettersson, Nino Runeberg, Jan Lundell and Markku Räsänen in August of 2000. Stable only at very low temperatures, argon fluorohydride begins to decompose once it warms above -246°C (-411°F). Because of this limitation, argon fluorohydride has no uses outside of basic scientific research.
Estimated Crustal Abundance: 3.5 milligrams per kilogram
Estimated Oceanic Abundance: 4.5×10-1 milligrams per liter
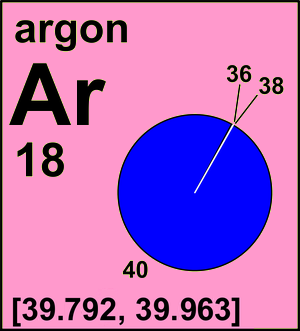
Number of Stable Isotopes: 3 (View all isotope data)
Ionization Energy: 15.760 eV
Oxidation States: 0
Electron Shell Configuration: | 1s2 |
2s2 2p6 | |
3s2 3p6 |
For questions about this page, please contact Steve Gagnon.
Argon Atomic Mass
What is the electron configuration of argon?
1 Answer
The electronic configuration of Argon (atomic number is 18) is-
Note:-
For writing the electronic configuration of elements, the Aufbau Principle is used. In Aufbau Principle, the electrons are filled according to the increasing energy level of orbitals.
Argon Atomic Weight
According to the Aufbau Principle, first the atomic number of element is determined (like here oxygen has atomic number 8) and then the electrons are filled in this order-
1s 2s 2p 3s 3p 4s 3d and so on..
.
In 's' orbital, maximum number of filling the electrons is 2.
In 'p' orbital, maximum number of filling the electrons is 6.
In 'd' orbital, maximum number of filling the electrons is 10.
In 'f' orbital, maximum number of filling the electrons is 14.
For example-
i) The electronic configuration of Chlorine (atomic number is 17) is-
ii) The electronic configuration of Titanium (atomic number is 22) is-
Related questions
